The Artificial Intelligence in Medicine Laboratory (AIMLab.) researches innovative pattern recognition algorithms to exploit the information encrypted within large datasets of physiological time series. AIMLab. leverages these new data-driven algorithms toward the creation of novel intelligent remote patient monitoring systems.
With billions of mobile devices worldwide, the low cost of mobile-connected medical sensors, and the widespread availability and use of medical imaging apparatuses, recording and transmitting medical data has become easier than ever. However, this ‘wealth’ of medical data has had limited success in providing novel actionable clinical information to improve patient care within and outside the traditional clinical environment. This is due to the lack of robust intelligent algorithms that can exploit the information encrypted within these ‘big databases’ of physiological time-series and medical images and take individual variability into account. Exploiting these datasets necessitates an in-depth understanding of the physiology underlying the patterns present in the medical time series and images, and the use of advanced digital signal processing and machine learning tools (e.g., deep learning) to recognize and extract patterns characteristic of health function in order to translate these patterns into clinically actionable information. The Artificial Intelligence in Medicine Laboratory (AIMLab.) researches innovative pattern recognition algorithms to exploit the information encrypted within large datasets of physiological time series and images. AIMLab. leverages these new data-driven algorithms toward the creation of novel intelligent patient monitoring systems. See this short video presenting the lab students and some of our projects.
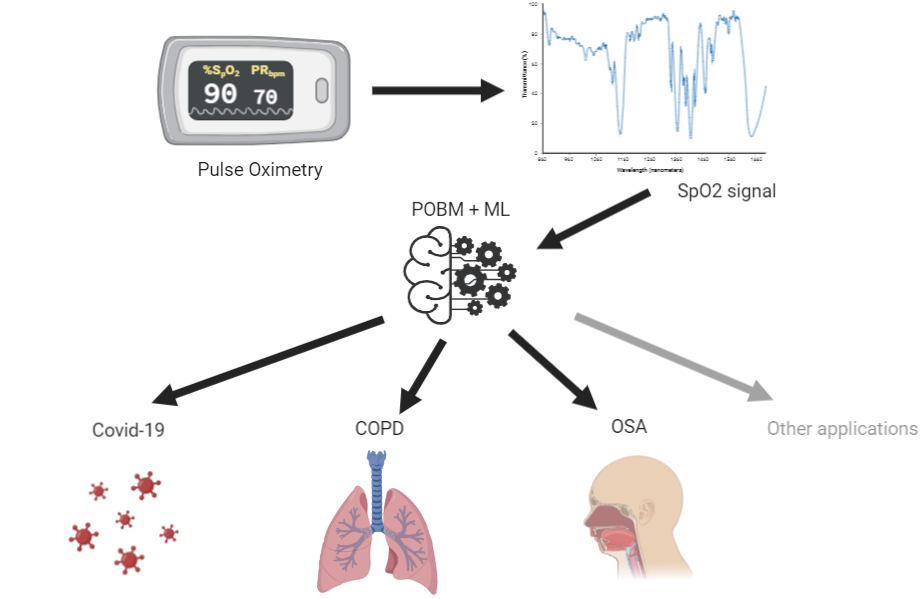
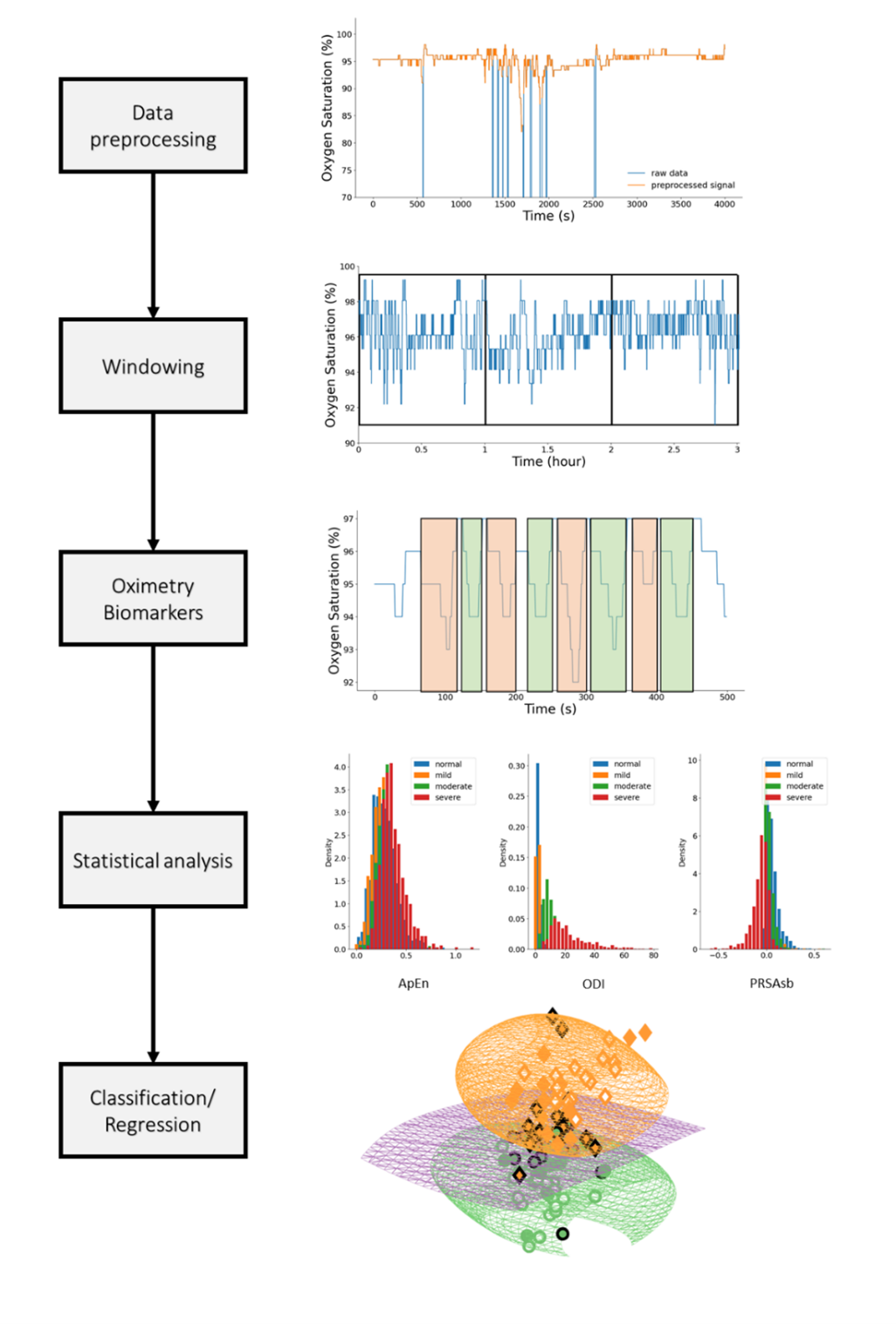
Sleep is essential for human health, well-being, and longevity. Insufficient sleep and poor sleep quality are known to cause a myriad of physical and mental diseases such as cardiovascular disease, obesity and depression. Sleep disorders are highly prevalent, affecting up to one-sixth of the global adult population. Furthermore, during sleep individuals remains more still than during wakefulness. This enables hours of data acquisition with reduced noise, overall providing longer recordings. The long and quality recordings may then be automatically analyzed by artificial intelligence algorithms to identify patterns of sleep or other diseases. This means, sleep can be regarded as a ‘testbed’ for physiological functions. We recently coined this paradigm ‘medicine during sleep’. We hypothesized that some conditions may be better expressed during sleep thus providing an added prognosis value. Therefore, sleep may be a particularly suitable time for performing diagnosis and monitoring of conditions such as some cardiovascular and respiratory diseases. Consequently, this research focuses on the development of artificial intelligence algorithms trained on large databases for the purpose of remote diagnosis and monitoring of sleep and other conditions by leveraging long term physiological time series recorded overnight.
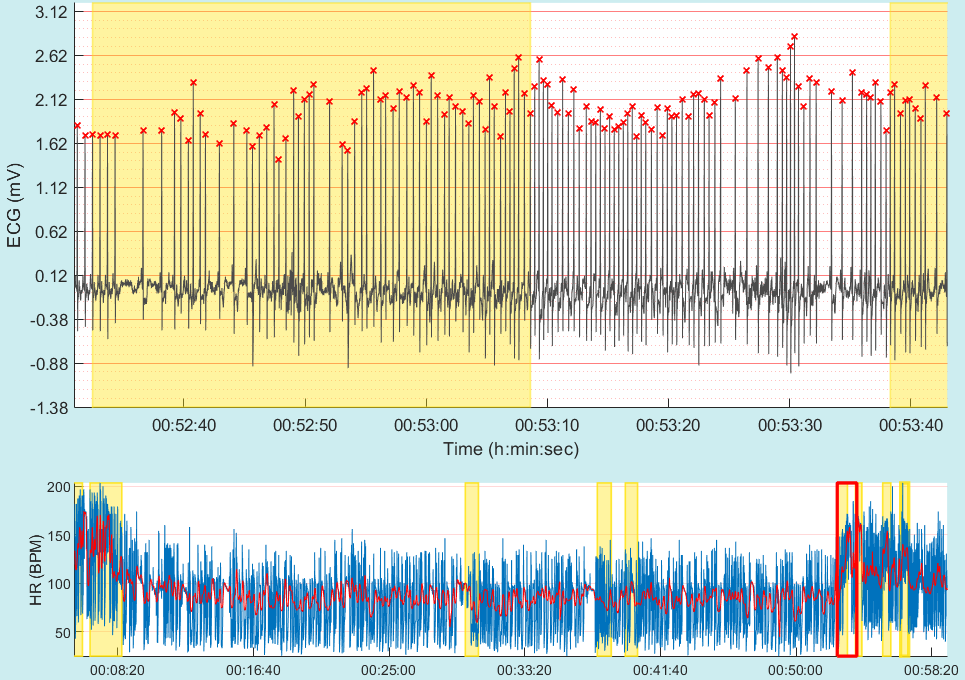
Atrial fibrillation (AF) is the most prevalent heart arrhythmia. It is associated with a 5-fold increase in stroke incidence and a 3.5-fold mortality risk increase. Across ethnicities, ages and sexes AF has been reported to have varying prevalence. A recent Swedish study reported on 89 out of 908 (9%) false positive automated misdiagnoses of AF, with almost half of these cases not corrected by the overreading primary-care physician, sometimes leading to inappropriate treatment with anticoagulant therapy. Machine learning presents a unique opportunity to provide an accurate automated diagnosis of AF. Yet, these models must demonstrate generalizability to external datasets integrating a range of population samples. This research intends to develop new machine learning models to support risk prediction and the diagnosis of AF from long term electrocardiogram (ECG) recordings.
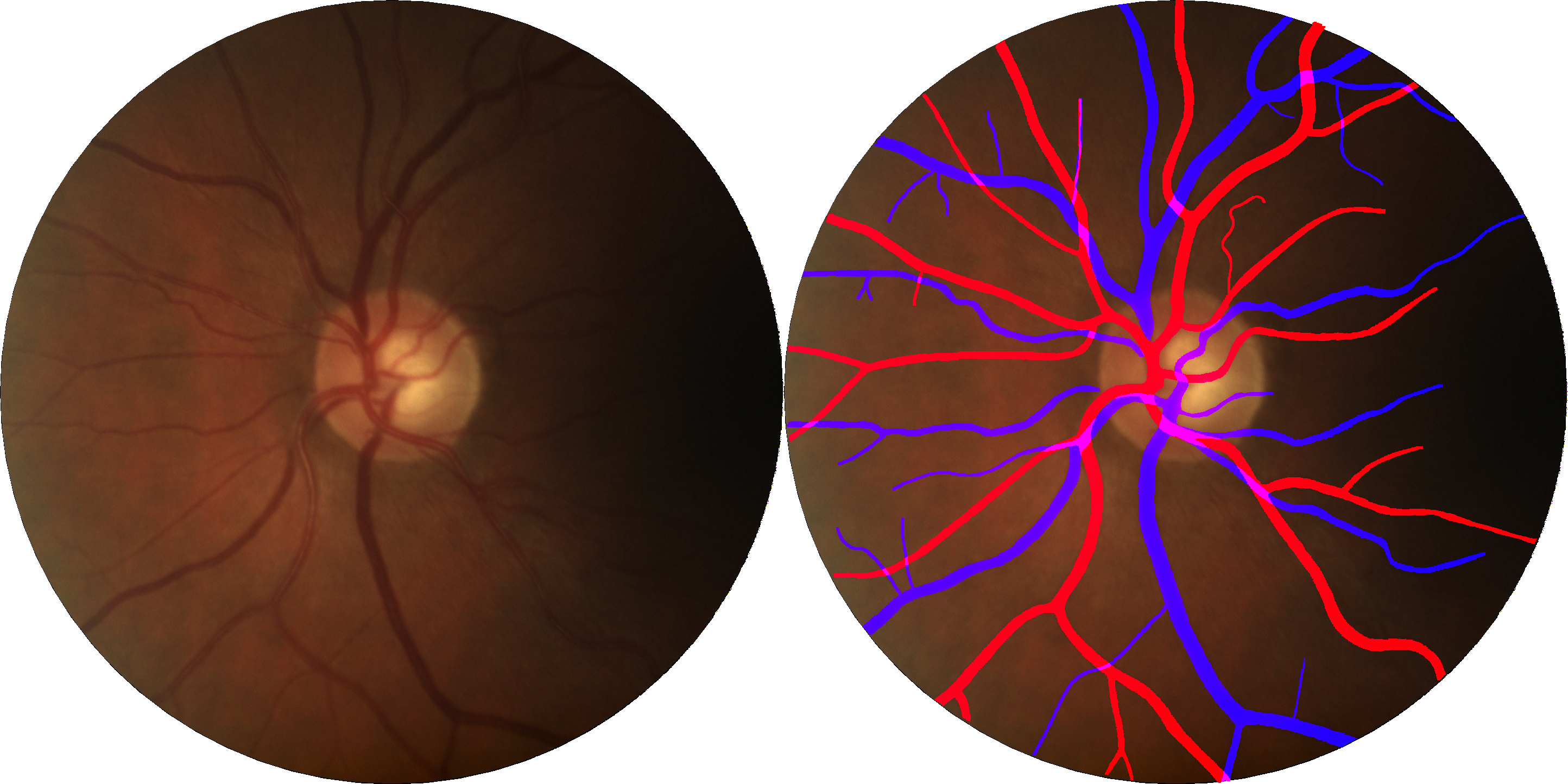
According to the National Center for Health Statistics, cardiovascular diseases (CVD), including coronary heart disease and stroke, are the most common cause of death in the USA. Research has shown that retinal microvascular abnormalities are present in CVD. Retinal vasculature can be non-invasively assessed using digital fundus images (DFI), which can be easily obtained using a fundus camera. Consequently, retinal vascular features obtained from DFI may be used to characterize and analyze vascular health. In order to clinical machine learning decision support system, it is necessary to fully automate the computation of these biomarkers from the segmented vasculature. Accordingly, this research aims to develop machine learning algorithms to segment the vasculatures of DFI (venules and arterioles), engineer vasculature biomarkers and models that can diagnose or risk predict the occurrence of a CVD.